A Randomized, Placebo‐Controlled, Multiple‐Ascending‐Dose Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Soluble Guanylate Cyclase Stimulator Praliciguat in Healthy Subjects
Clinical Pharmacology in Drug Development (2018)
The results of a Phase 1b study of praliciguat in healthy volunteers support its evaluation for the treatment of conditions associated with deficient nitric oxide signaling.
Demonstration of praliciguat target engagement and its effects on cGMP in human volunteers
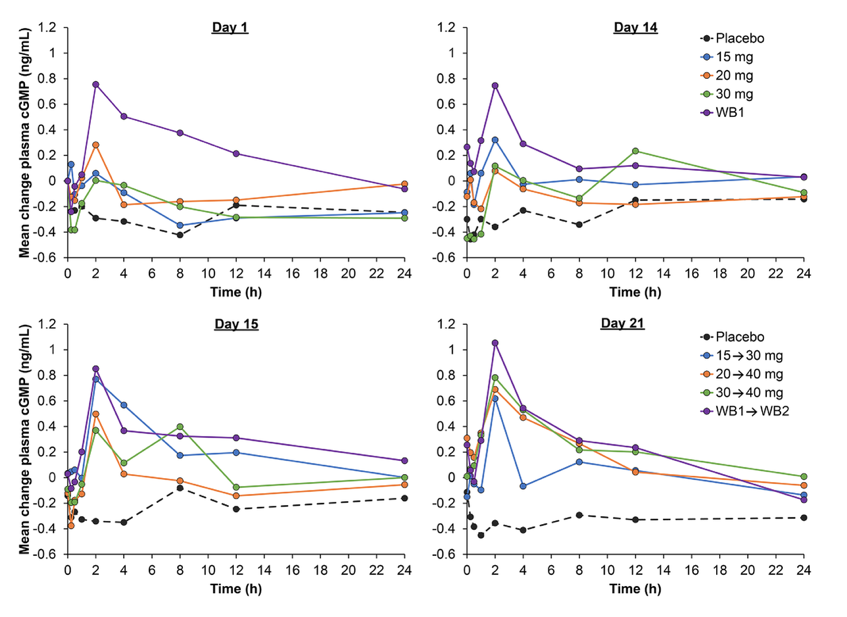
Effects of praliciguat on cGMP over 24 hours. Plots are mean change from day 1 predose baseline at starting (lower) doses on days 1 and 14, and at up‐titration (higher) doses on days 15 and 21. WB1, weight‐based dosing for the first 14 days (maximum of 25, 30, 35, or 40 mg, but not to exceed 0.5 mg/kg); WB2, weight‐based dosing for days 14–21 (40 mg or ≤0.5 mg/kg for subjects with baseline weight ≥ 90 kg). Hanrahan et al. 2018. Clinical Pharmacology in Drug Development, First published: 13 November 2018, DOI: (10.1002/cpdd.627).

 Return to Archive
Return to Archive