Fourteen-Day Study of Praliciguat, a Soluble Guanylate Cyclase Stimulator, in Patients with Diabetes and Hypertension
American Diabetes Association 78th Scientific Sessions (2018)
This oral presentation, delivered at the American Diabetes Association 78th Scientific Sessions, summarizes the results from an exploratory Phase 2a clinical study of praliciguat in patients with diabetes and hypertension.
Praliciguat-treated patients had greater declines in fasting plasma glucose than placebo-treated patients
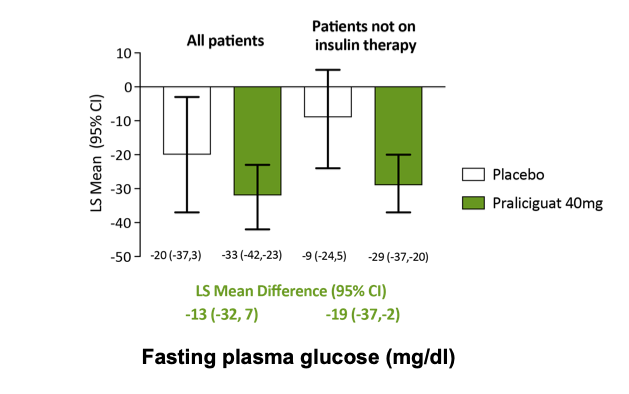
Least squares (LS) mean changes from baseline values with 95% confidence intervals (CI) shown beneath each bar. LS mean differences between praliciguat and placebo-treated patients with 95% CI are shown below each pair of bars. All patients: placebo n=6, praliciguat 40 mg n=19. Patients not on insulin therapy: placebo n=4, praliciguat 40 mg n=11. Hanrahan et al. (ADA 2018) oral presentation
See also: An oral presentation from the EASD Annual Meeting evaluating the results of praliciguat treatment in subgroups of this Phase 2a study

 Return to Archive
Return to Archive